Medical masks are usually called Surgical Mask or Procedure Mask in English, and may also be called Dental Mask, Isolation Mask, Medical Face Mask, etc. In fact, they are the same. The name of the mask does not indicate which protective effect is better.
Although various English nouns actually refer to medical masks, there are often different styles. The traditional surgical masks used in the operating room are “Tie-On” bandages (left in the above picture), so many are called surgical masks. Surgical masks are also designed with straps. For ordinary people, the “Earloop” ear-hook (right in the picture above) medical mask will be more convenient to use.
Quality standards for medical surgical masks
Medical surgical masks in the United States are subject to FDA approval and require certain particle filtration efficiency, fluid resistance, flammability data, etc., in order to meet the standards. So what are the standard requirements for medical surgical masks? FDA requires medical masks to provide the following test data:
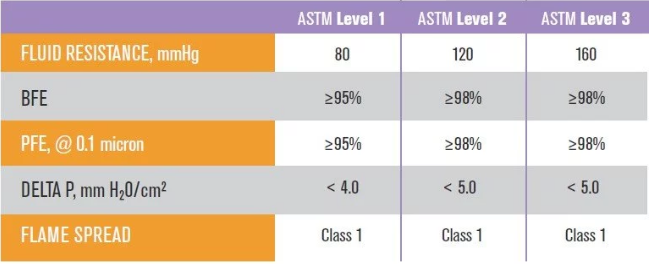
• Bacterial Filtration Efficiency (BFE / Bacterial Filtration Efficiency): an indicator that measures the ability of medical masks to prevent the passage of bacteria in droplets. The ASTM test method is based on a biological aerosol with a size of 3.0 microns and containing Staphylococcus aureus. The number of bacteria can be filtered out by the medical mask. It is expressed as a percentage (%). The higher the percentage, the stronger the ability of the mask to block bacteria.
• Particulate Filtration Efficiency (PFE / Particle Filtration Efficiency): measures the filtering effect of medical masks on sub-micron particles (virus size) with a pore size between 0.1 microns and 1.0 microns, also expressed as a percentage (%), the higher the percentage, the The better the mask’s ability to block viruses. The FDA recommends using non-neutralized 0.1 micron latex balls for testing, but larger particles can also be used for testing, so pay attention to whether “@ 0.1 micron” is marked after PFE%.
• Fluid Resistance: It measures the ability of surgical masks to resist the penetration of blood and body fluids. It is expressed in mmHg. The higher the value, the better the protection performance. The ASTM test method is to use artificial blood to spray at three levels of pressure: 80mmHg (venous pressure), 120mmHg (arterial pressure) or 160mmHg (potential high pressure that may occur during trauma or surgery) to see if the mask can block the flow of liquid from the outer layer to Inner layer.
• Differential Pressure (Delta-P / pressure differential): measures the air flow resistance of medical masks, visually displays the breathability and comfort of medical masks, in mm H2O / cm2, the lower the value, the more breathable the mask.
• Flammability / Flame Spread (flammability): Because there are many high-energy electronic medical equipment in the operating room, there are many potential ignition sources, and the oxygen environment is relatively sufficient, so as a surgical mask must have a certain flame retardancy.
Through the BFE and PFE tests, we can understand that ordinary medical masks or surgical masks have certain effects as epidemic prevention masks, especially to prevent some diseases that are mainly spread by droplets; but medical masks cannot filter tiny particles in the air. It has little effect on preventing bacteria and airborne diseases that can be suspended in the air.
ASTM Standards for Medical Surgical Masks
ASTM Chinese is called the American Society for Testing and Materials. It is one of the largest international standardization organizations in the world. It specializes in researching and formulating material specifications and test method standards. The FDA also recognizes ASTM test methods for surgical masks. They are tested using ASTM standards.
ASTM’s evaluation of medical surgical masks is divided into three levels:
• ASTM Level 1 Lower Barrier
• ASTM Level 2 Moderate Barrier
• ASTM Level 3 High Barrier
It can be seen from the above that the ASTM test standard uses 0.1 micron particles to test the filtration efficiency of PFE particles. The lowest Level 1 medical mask must be able to filter bacteria and viruses carried in 95% or more of the droplets, and the more advanced Level 2 and Level 3 medical masks can filter bacteria and viruses carried by 98% or more of the droplets. The biggest difference between the three levels is fluid resistance.
When buying medical masks, friends should look at the certification standards written on the packaging, which standards are tested, and what standards are met. For example, some masks will simply say “Meets ASTM F2100-11 Level 3 Standards“, which means that they meet the ASTM Level 3 / High Barrier standard.
Some products may also specifically list each measurement value. The most important thing to prevent the virus is “PFE% @ 0.1 micron (0.1 micron particle filtration efficiency)”. As for the parameters that measure the fluid resistance and flammability of the blood splash, Whether the highest level of standards has little effect.
CDC Anti-epidemic Mask Description
Medical surgical masks: not only prevent the wearer from spreading the germs, but also protect the wearer from spray and liquid splashes, and have a preventive effect on diseases spread by large particles of spray; but ordinary medical masks cannot filter small Particulate aerosol has no preventive effect on airborne diseases.
N95 masks: can block large particles of droplets and more than 95% of non-oily small particle aerosols. Properly wearing NIOSH certified N95 masks can prevent airborne diseases and can be used as the lowest level of protective masks for airborne diseases such as TB tuberculosis and SARS However, N95 masks cannot filter gas or provide oxygen, and are not suitable for toxic gas or low oxygen environments.
Surgical N95 masks: meet N95 particle filtration standards, prevent droplets and airborne diseases, and block blood and body fluids that may occur during surgery. FDA approved for surgical masks.
Post time: May-25-2020